Introduction:
Cancer-associated thrombosis (CAT) is a leading cause of morbidity and mortality among patients with malignancies. Direct oral anticoagulants (DOACS) are frequently used to manage CAT. Bleeding risk is higher in cancer patients influencing the risk/benefit balance of treatment. Unlike in other cases of venous thromboembolism (VTE), there are no guidelines for dose reduction and extended prophylaxis after the initial period of full-dose treatment for cancer patients. We aimed to provide efficacy and safety data on patients with CAT that received extended VTE prophylaxis with a reduced dose of apixaban for the prevention of VTE recurrence.
Aim:
To investigate the clinical outcomes of patients with CAT treated with low-dose apixaban after initial treatment with full-dose anticoagulation.
Methods:
Data of all cancer patients over the age of 18 years from Sheba medical center that were diagnosed with VTE during the years 2012-2022, and treated with low-dose apixaban were retrieved using the MD Clone platform. All demographic, laboratory, and clinical data were collected from computerized medical files and statistically analyzed. The study was approved by the local IRB committee.
Results:
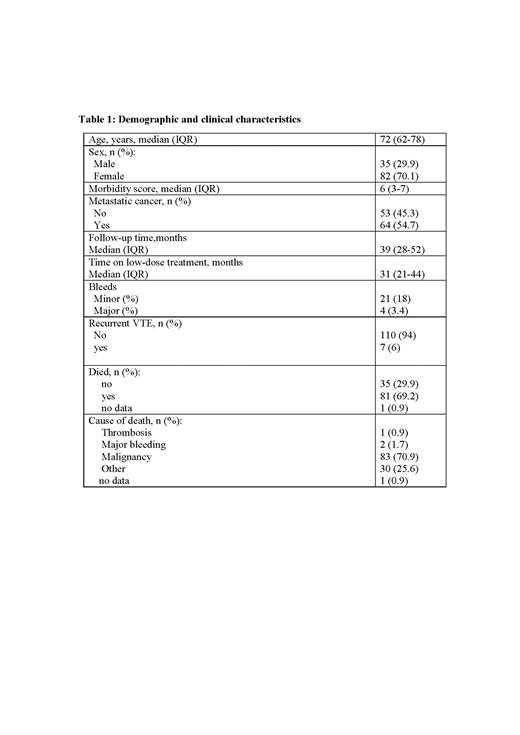
One hundred and seventeen patients with CAT, treated by a reduced dose of apixaban after initial full dose treatment for at least 6 months, were followed at our center. Table 1 summarizes the demographic and clinical characteristics of our cohort.
At the time of diagnosis with CAT the median age of patients was 72 (IQR 62-78).70.1% were female and 29.9% were male. 54.7% of patients had metastatic disease. After initial treatment with full-dose anticoagulation, patients were switched to low-dose apixaban for a median of 31 months (IQR 21-44 months). Twenty-one patients (18%) had a minor bleed and four patients (3.4%) had a major bleed while treated with low-dose apixaban. During this period 7 patients (6%) had a recurrent VTE. Two-thirds of the patients died. Mortality was attributed to thrombosis in one (0.9%) patient and major bleeding in two patients (1.7%).
Conclusions:
Low-dose apixaban may be a safe and appropriate option for extended treatment of cancer patients with VTE, with low rates of recurrence and major bleeding.
OffLabel Disclosure:
Lalezari:Pfizer: Honoraria. Kenet:BSF, Opko Biologics, Pfizer, Roche, Shire: Research Funding; Bayer, BioMarin, BPL, CSL, Pfizer, Novo Nordisk, Roche, Sanofi- Genzyme, Sobi, Spark, Takeda, Uniqure: Honoraria; ASC Therapeutics, Bayer, BioMarin, Novo Nordisk, Pfizer, Roche, Sanofi- Genzyme, Sobi, Takeda, Uniqure: Consultancy; Sheba Medical Center and Sackler Faculty of Medicine, Tel Aviv University: Current Employment; PedNet foundation: Membership on an entity's Board of Directors or advisory committees. Levy-Mendelovich:Roch LTD: Honoraria; Pfizer: Honoraria, Research Funding; Novo nordisk: Honoraria, Research Funding.
Apixaban is used for treating patents with cancer associated thrombosis- however reduced dose in not common treatment

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal